Know all about the concept of Biomolecules | Chemistry
- Other Laws|Blog|
- 5 Min Read
- By Taxmann
- |
- Last Updated on 22 September, 2022
Table of Contents
1.3 Reaction of carbohydrates with phenylhydrazine
1.5 Reducing and non-reducing sugars
Check out Tan Print's Chemistry for NTA CUET (UG) 2022 which intends to cater to the principal needs of all the students preparing for the Common University Entrance Test (CUET) at the Undergraduate Level in the Chemistry Domain. This book contains the practice material in a highly student-friendly and thorough manner.
1. Carbohydrate
1.1 D-(+) – Glucose
Glucose is prepared by the hydrolysis of sucrose, while commercially it is prepared by the hydrolysis of starch.
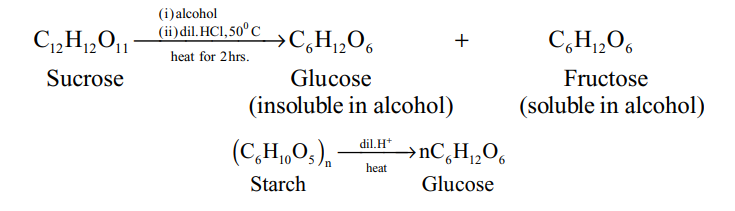
-
- Glucose is a white crystalline substance
- Soluble in water, sparingly soluble in alcohol.
- It is dextrorotatory, hence also called dextrose.
- It shows mutarotation (change in specific rotation in aqueous solution of the compound).
1.2 Chemical properties
(i) 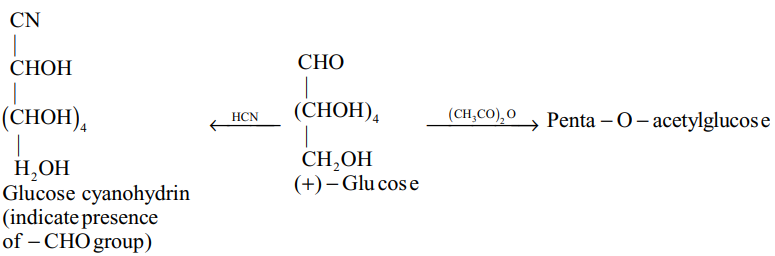
(ii) 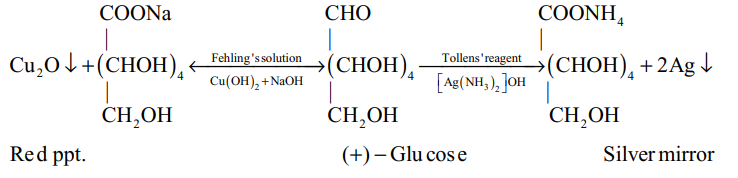
(iii) 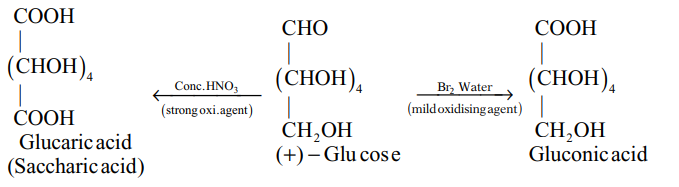
(iv) 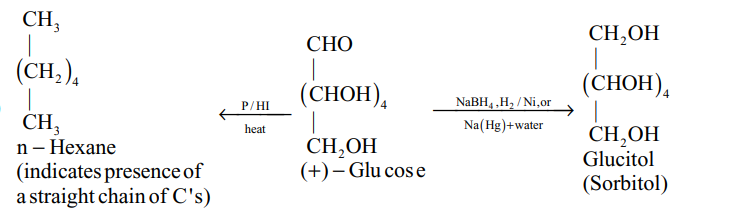
(v) 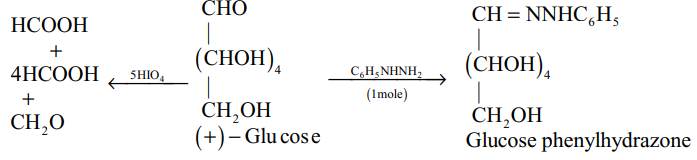
1.3 Reaction of carbohydrates with phenylhydrazine
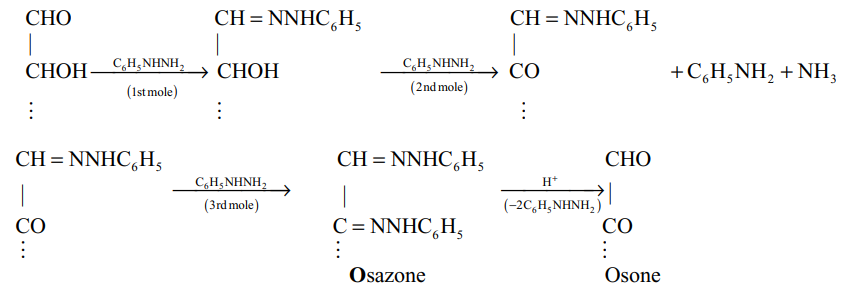
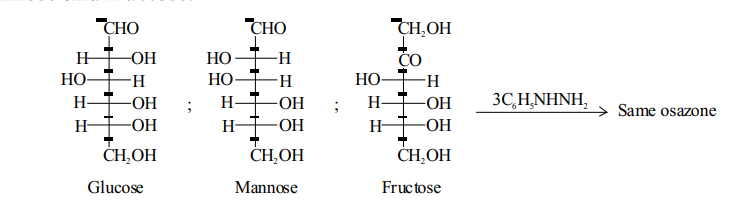
Both of the phenylhydrazine residues of osazone can be removed to form dicarbonyl compounds, known as osones.
Osazones are yellow coloured, crystalline compounds. osazone formation involves only the first two carbon atoms, i.e. CHOCHOH- in aldoses and in ketoses, of a compound without affecting the configuration of the rest of the molecule. Thus hexoses having similar configuration on and will form same osazone, e.g. glucose, mannose and fructose.
Tests of glucose
(i) Molisch’s test : This is a general test for carbohydrates. A drop or two of alcoholic solution of a-naphthol is added to 2 mL of glucose solution. concentrated is added carefully along the sides of the test tube. The formation of a violet ring, at the junction of two liquids confirms the presence of a carbohydrate.
(ii) Silver mirror test : A mixture of glucose and ammonical silver nitrate is warmed in a test tube. Appearance of silver mirror on the inner walls confirms glucose.
(iii) Fehling’s solution test : Glucose is warmed with Fehling’s solution. A red precipitate of cuprous oxide is formed.
(iv) Osazone formation : Glucose on heating with excess of phenylhydrazine gives a yellow crystalline compound.
Structure of Glucose
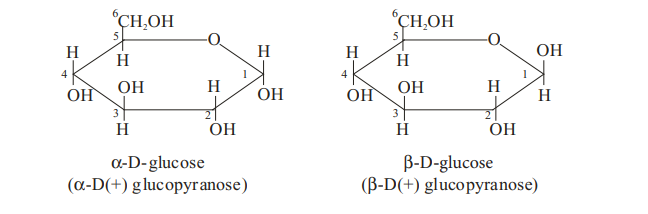
(i) Glucose exists in two stereoisomeric forms (a and b). a -glucose with specific rotation +1100 , whereas b-glucose with specific rotation +19.70
(ii) A aqueous solution of glucose shows mutarotation, i.e., its specific rotation gradually decreases from +1100 to 52.50 in case of a-glucose and increases from 19.70 to 52.50 in case of b-glucose.
The two forms of D-glucose are also shown by Haworth projection formula which are given below :
1.4 Disaccharides
The disaccharides yield on hydrolysis two monosaccharides.
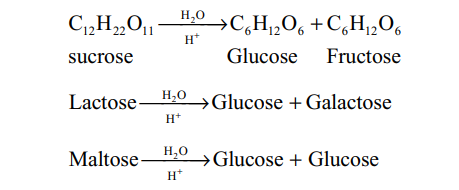
Sucrose or cane-sugar (C12H22O11) : It is dextrorotatory but does not show mutarotaton. It is a non-reducing sugar as it does not reduce Tollen’s or Fehling’s reagent.
It is composed of a-D-glucopyranose unit and a b-D-fructofuranose unit.
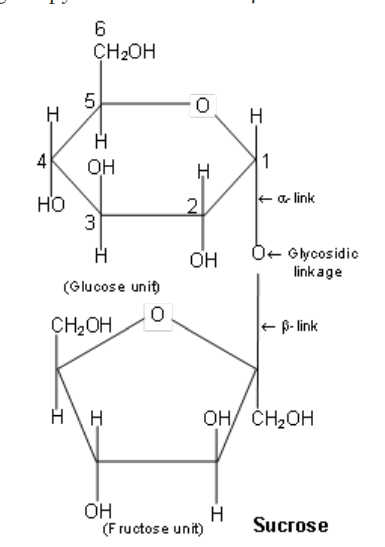
These units are joined by a-b-glycosidic linkage between C-1 of the glucose unit and C-2 of the fructose unit.
Inversion of cane-sugar
The hydrolysis of sucrose by boiling with a mineral acid or by enzyme invertase or sucrose, produces a mixture of equal molecules of D(+) glucose and D(-) fructose.
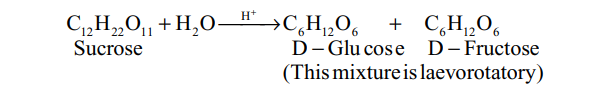
Thus, in the process of hydrolysis of sucrose, is termed as inversion of sugar and the hydrolysed mixture having equal molar quantities of D(+) glucose and D(-) fructose is called invert sugar. The enzyme that brings the inversion is named as invertase.
1.5 Reducing and non-reducing sugars
| Reducing sugars | Non-reducing sugars |
| D-Glucose, D-Fructose, | Sucrose, Starch |
| Maltose, Lactose | Glycogen, Cellulose |
1.6 Amino Acids
Amino acids : An amino acid is a bifunctional organic molecule that contains both a carboxyl group, -COOH, as well as an amine group, .
In an a – amino acid, the amine group is located on the carbon atom adjacent to the carboxyl group (the a -carbon atom). The general structure of the a -amino acids is represented as :
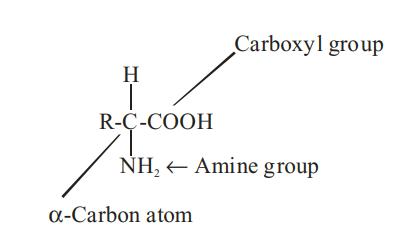
Methods of Preparation of a–Amino Acids
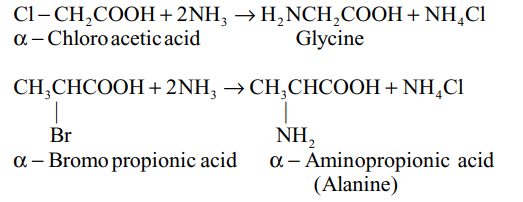
(i) Amination of a –halo acids :
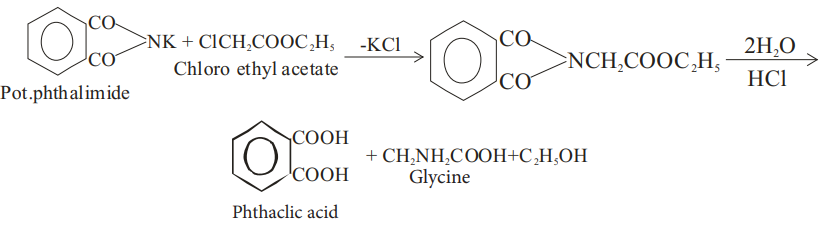
(ii) Gabriel phthalimide synthesis :
(iii) Knoop synthesis :
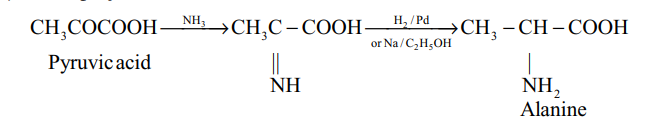
(iv) Strecker synthesis :
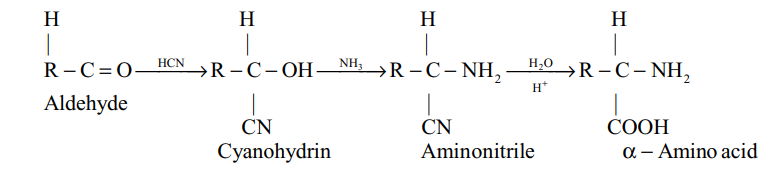
Properties :
(i) Except glycine, all the a -amino acids are optically active.
(ii) Zwitter ion and isoelectric point : the – NH2 group is basic and –COOH group is acidic, in neutral solution, it exists in an internal ionic form called a Zwitter ion, also known as dipolar ion.
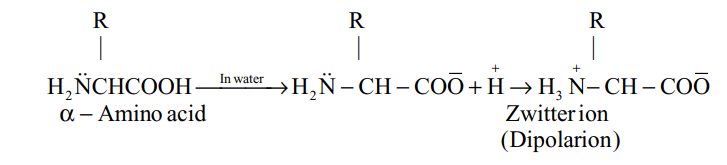
The Zwitter ion is dipolar, charged but overall electrically neutral and contains both a positive and negative charge.
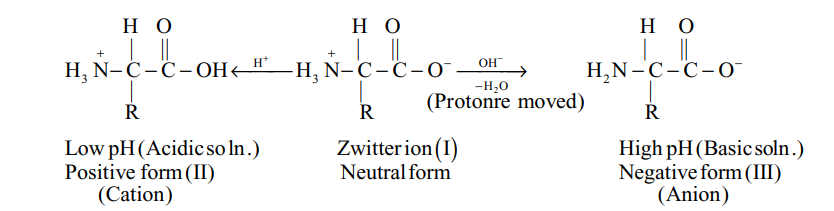
When an ionised form of amino acid is placed in an electric field, it will migrate towards the opposite electrode. Depending on the pH of the medium, following three things may happen :
(i) In acidic solution (low pH), the positive ion moves towards cathode [exist as cation, structure (II)].
(ii) In basic solution (high pH), the negative ion moves towards anode [exist as anion, structure (III)].
(iii) The Zwitter ion does not towards any of the electrodes [neutral dipolar ion, structure (I)].
The intermediate pH at which the amino acid shows no tendency to migrate towards any of the electrodes and exists the equilibrium when placed in an electric field is known as isoelectric point.
Disclaimer: The content/information published on the website is only for general information of the user and shall not be construed as legal advice. While the Taxmann has exercised reasonable efforts to ensure the veracity of information/content published, Taxmann shall be under no liability in any manner whatsoever for incorrect information, if any.

Taxmann Publications has a dedicated in-house Research & Editorial Team. This team consists of a team of Chartered Accountants, Company Secretaries, and Lawyers. This team works under the guidance and supervision of editor-in-chief Mr Rakesh Bhargava.
The Research and Editorial Team is responsible for developing reliable and accurate content for the readers. The team follows the six-sigma approach to achieve the benchmark of zero error in its publications and research platforms. The team ensures that the following publication guidelines are thoroughly followed while developing the content:
- The statutory material is obtained only from the authorized and reliable sources
- All the latest developments in the judicial and legislative fields are covered
- Prepare the analytical write-ups on current, controversial, and important issues to help the readers to understand the concept and its implications
- Every content published by Taxmann is complete, accurate and lucid
- All evidence-based statements are supported with proper reference to Section, Circular No., Notification No. or citations
- The golden rules of grammar, style and consistency are thoroughly followed
- Font and size that’s easy to read and remain consistent across all imprint and digital publications are applied



 CA | CS | CMA
CA | CS | CMA
